
This webpage is intended for UK Healthcare Professionals only.
Prescribing information and adverse event reporting can be found at the bottom of this page
EFFICACY YOU CAN SEE, SATISFACTION YOU CAN FEEL1
KLISYRI®(tirbanibulin) is indicated for the field treatment of non-hyperkeratotic, non-hypertrophic actinic keratosis (Olsen grade 1) of the face or scalp in adults.2
AN IDEAL TREATMENT EXPERIENCE FOR AK PATIENTS3
Up to 97.1% of patients achieved complete/partial clearance.*3

Generally well tolerated.4
Works with just 5 days of treatment.2

87% of dermatologists and patients were willing to use KLISYRI® again.5
*Real World Evidence results from an Italian multi-centre phase IV study. A satisfactory response was observed in 34/35 (97.1%) and complete clearance in 19/35 (54.3%) patients with Olsen grade I lesions of the face and scalp area controlled more than 8 weeks after treatment out of 111 patients assessed with Olsen I lesions.1
NOVEL MoA
KLISYRI®, a topical therapy with a novel mechanism of action6-9
KLISYRI® is a novel inhibitor of tubulin polymerisation that induces cell cycle arrest and, ultimately, apoptotic cell death6-8
These events promote a potent antiproliferative effect resulting in clearance of the lesions through mainly mild or moderate LSRs which are resolving spontaneously6-9
Press play to see for yourself how KLISYRI® delivers strength with tolerability.
KLISYRI®’S TREATMENT RESULTS IN A REGULAR EPIDERMAL ARCHITECTURE10-11


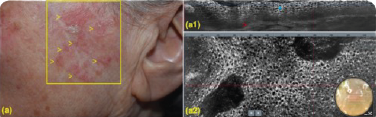

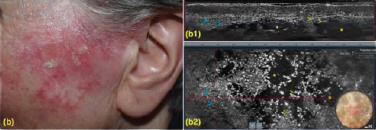

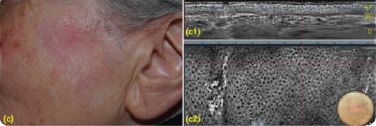
Addressing all AK lesions and field cancerisation helps reduce recourrance and progression to SCC.11
Clinical and LC-OCT (DeepLive, DAMAE Medical) evaluation were performed at baseline and after 8 and 57 days, 57,87% of the lesions showed clinical clearance10
(a1) LC-OCT vertical section showing hyperkeratosis (yellow asterisks), parakeratosis (yellow arrow), acanthotic, irregular epidermis (blue asterisks) and preserved dermal-epidermal junction (red arrow).
(a2) LC-OCT horizontal section at the level of the stratum spinosum showing keratinocytes variability in shape and size. (b1) LC-OCT vertical section showing large, ovoidal dark spaces (vesicles, yellow asterisks), targeted/ring-like cells (apoptotic keratinocytes, yellow arrows) and small, round bright elements (inflammatory cells, blue arrows). (b2) LC-OCT horizontal section at the level of the stratum spinosum showing large, ovoidal dark spaces (vesicles, yellow asterisks), targeted/ring-like cells (apoptotic keratinocytes, yellow arrows) and small, round bright elements (inflammatory cells, blue arrows). (c1) LC-OCT vertical section showing a regular epidermal architecture. (c2) LC-OCT horizontal section at the level of the stratum spinosum showing a normal honeycomb pattern. Dermoscopic round inserts: the blue rectangle corresponds to the area examined by horizontal and vertical LC-OCT.
AK CLEARANCE
KLISYRI® applied once-daily for just five days offers significant complete AK clearance at day 572,4 in 49% of patients.
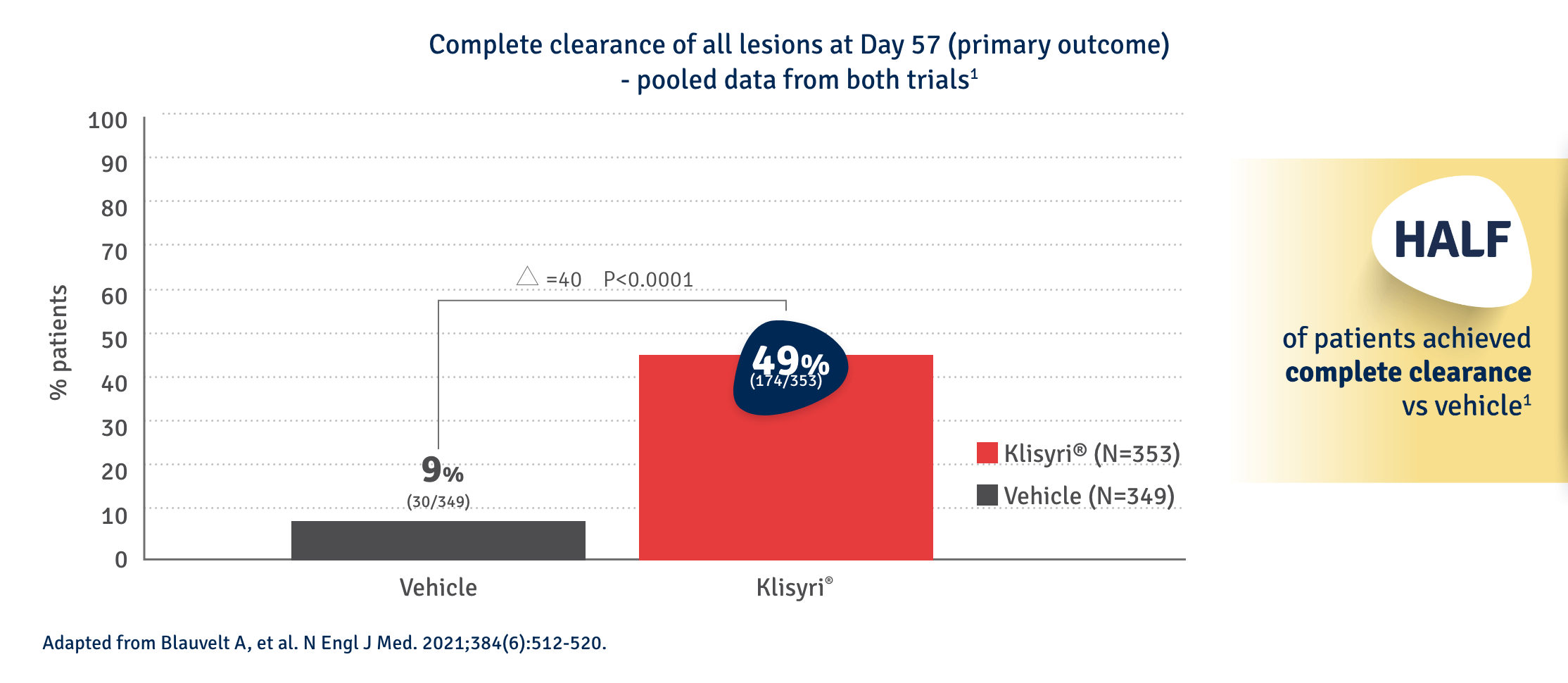
FACE*
*vs vehicle: 6%-14% (p<0.001) | **vs vehicle: 2%-11% (p<0.001) (% range across both trials presented)
For the subgroup analyses (face and scalp) confidence intervals were not adjusted for multiple comparisons and no clinical conclusions can be drawn from this data.
KLISYRI® applied once-daily for just five days offers significant partial AK clearance at day 572,4
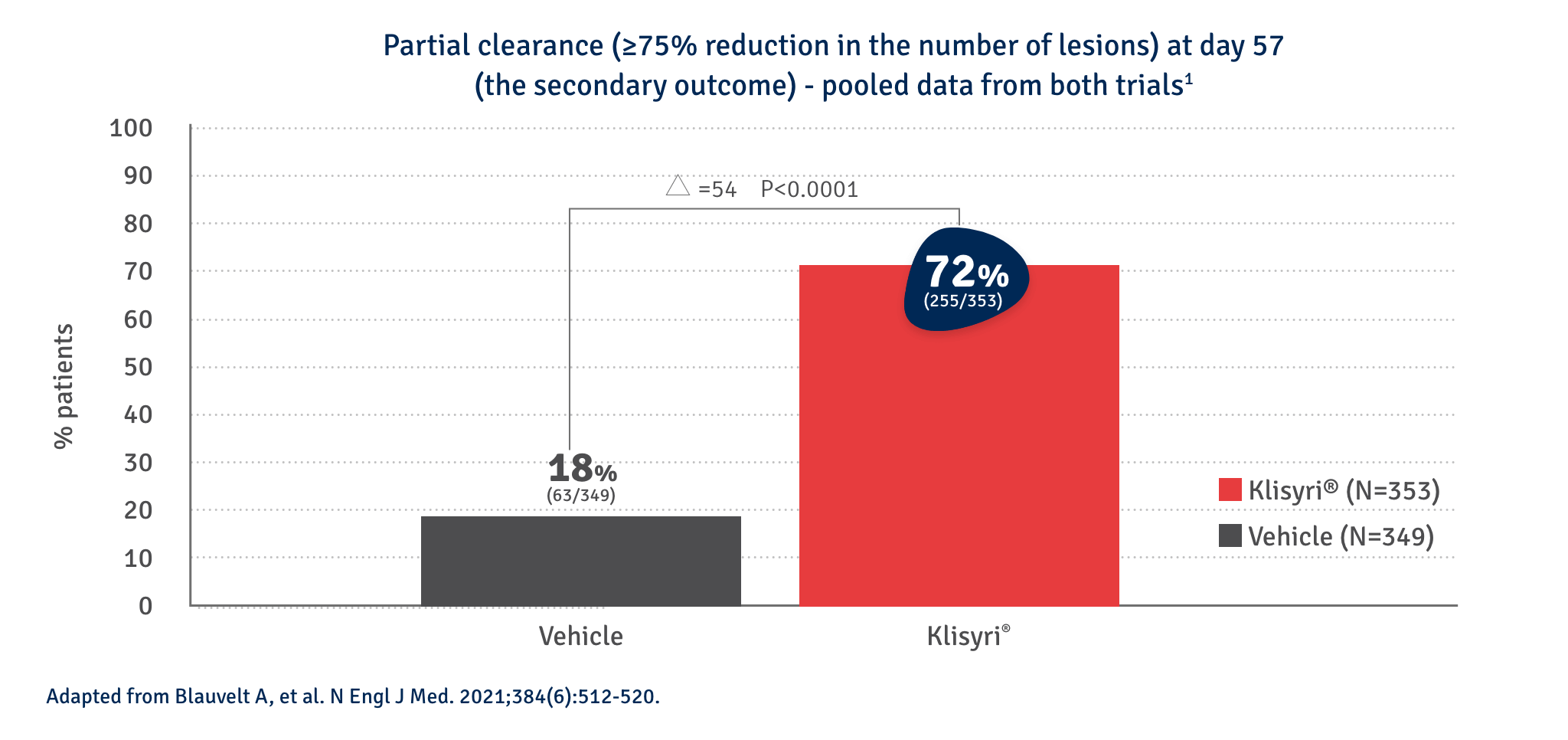

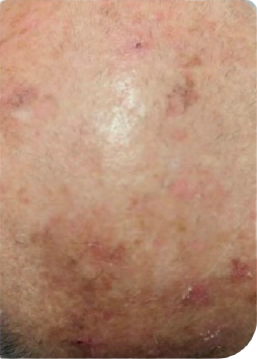
KLISYRI®’S SATISFACTORY RESPONSES ACHIEVED AFTER AN 8-WEEK TREATMENT FOLLOW-UP*3

Partial clearance*
Day 0
Follow up visit**
Clinical clearing
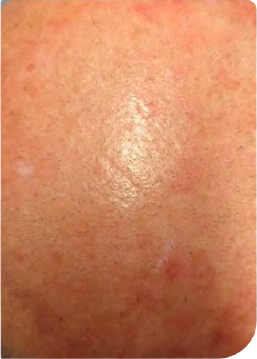
Male Patient, n.s years old AK on scalp

Complete clearance*
Day 0
Follow up visit**
Clinical clearing
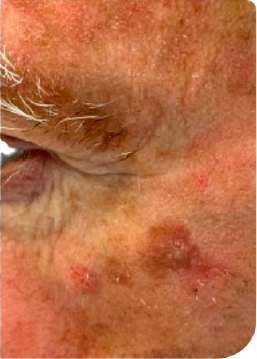
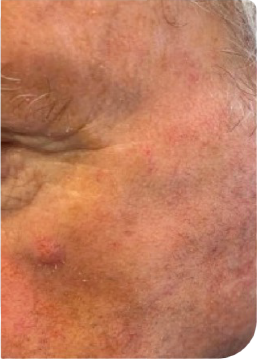
Male Patient, n.s years old AK on face
**Follow up visits after treatment application were performed at different time intervals (from 1 to more than 4 weeks), depending on availability in clinical practice.3
SAFETY PROFILE
KLISYRI® was generally well-tolerated, with no patients discontinuing use due to tolerability issues4
None of the patients’ local skin reactions (LSRs) required treatment and no patients discontinued use due to tolerability issues.4
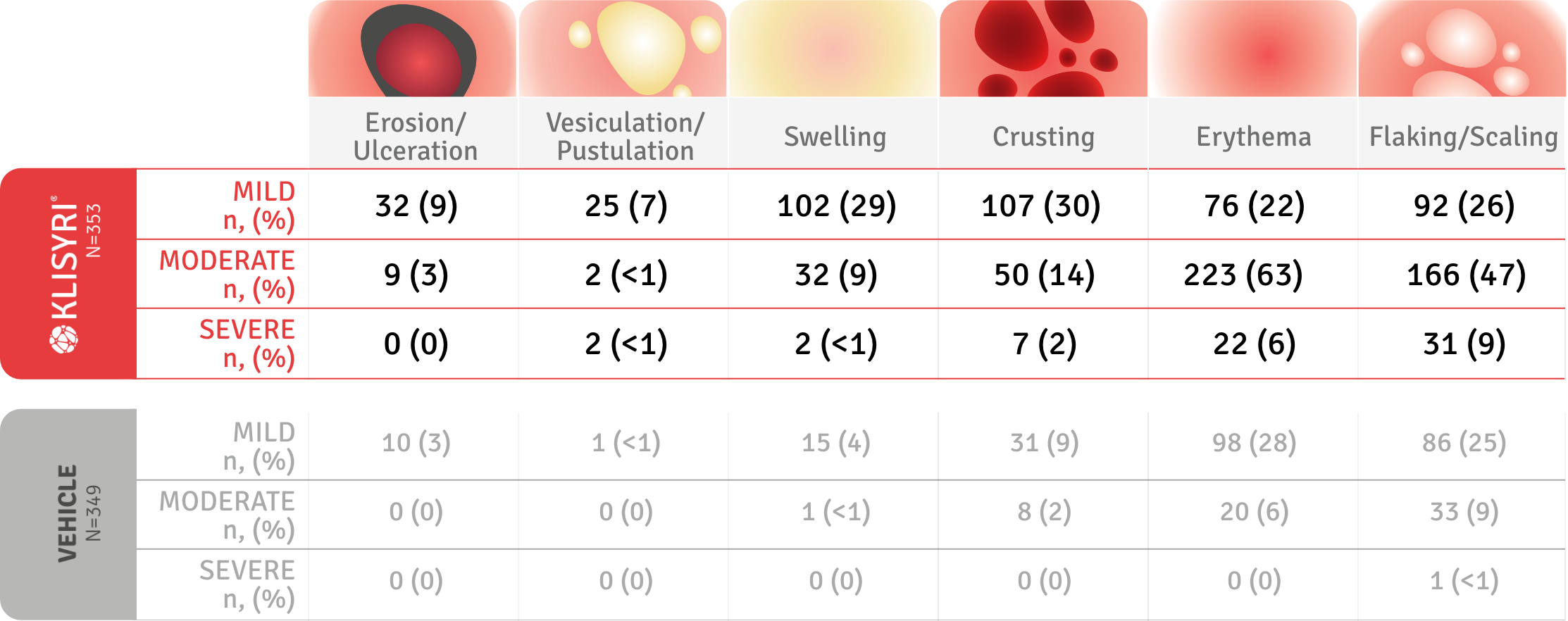
LSRs were graded as absent, mild (slightly or barely perceptible), moderate (distinct presence), and severe (marked or intense). Please consult the SPC for full information on LSRs
KLISYRI®, SHORT TREATMENT COURSE WITH JUST 5 DAYS OF TREATMENT2
Once a day for only 5 consecutive days2

KLISYRI® IN PRACTICE
PATIENT 1: with scalp lesions previously treated with other topicals12





THE PATIENT ACHIEVED COMPLETE LESION CLEARANCE AT D57 AND WAS WELL-TOLERATED, WITH SIGNIFICANT BENEFIT ON QUALITY OF LIFE1
PATIENT 2: with multiple lesions on forehead, previous history of skin cancer and already treated with cryotherapy and topical therapies13







AK LESIONS WERE COMPLETELY CLEARED AFTER 2 MONTHS, WITH NO CHANGES IN PIGMENTATION AND GLOBAL PATIENT SATISFACTION WITH THE TREATMENT RESULTS13
PCDS Guidelines
KLISYRI® (tirbanibulin) has received a strong recommendation from PCDS for Grade I lesions and small field change.7
AAD Guidelines
KLISYRI® (tirbanibulin) received a strong recommendation with a high certainty of evidence in the American Academy of Dermatology (AAD) Guidelines8
Guidelines of care for the management of actinic keratosis. J Am Acad Dermatol. 2022 Apr 18:S0190-9622(22)006 12-0
Recommendations for the Management of Actinic Keratosis with topical agents8
| Topical Field Treatment | Strength of Recommendation | Certainty of Evidence |
| Tirbanibulin | Strong | High |
| 5-Fluorouracil | Strong | Moderate |
| Imiquimod | Strong | Moderate |
| Diclofenac* | Conditional | Low |
Adapted from Eisen DB, et al. Focused update: Guidelines of care for the management of actinic keratosis. J Am Acad Dermatol. 2022 Apr 18:S0190-9622(22)00612-0. doi: 10.1016/j.jaad.2022.04.013. Online supplement.
*For patients with AKs, nonsteriodal anti-inflammatory drugs (NSAIDs) carry a black box warning for cardiovascular and gastrointestinal side effects.

Abbreviations
AE: adverse event; AKASI: Actinic Keratosis Area and Severity Index; AK: actinic keratosis; DEJ: dermo-epidermal junction; KN: keratinocyte nuclei; LC-OCT: line-field confocal optical coherence tomography; LSR: local skin reaction; MoA: Mode of Action; SC: stratum corneum; SCC: squamous cell carcinoma; VE: viable epidermis.
References
- Schlesinger T et al. Skin, 2023: 7(3); 771-787.
- KLISYRI® SmPC. Almirall.
- Nazzaro G, et al. International Journal of Dermatology. 2024; 63(11):1566-1574.
- Blauvelt A, et al. N Engl J Med. 2021;384(6):512-520.
- Gilaberte Y, et al. EJC Skin Cancer 2024; Volume 2, 100033.
- Smolinski MP, et al. J Med Chem 2018;61:4707-4719.
- Niu L, et al. J Biol Chem 2019;294(48):18099-18108.
- Blauvelt A, et al. SKIN The Journal of Cutaneous Medicine. 2020; 4(5), s63.
- Kempers S, et al. J Drugs Dermatol. 2020;19(11):1093-1100.
- Lacarrubba F, et al. J Eur Acad Dermatol Venereol. 2023 Sep;37(9):e1131-e1133.
- Schmitz L et al. J Eur Acad Dermatol Venereol. 2016; 30(8):1303-1307.
- Hadshiew I. Almirall symposium presented at the 31st EADV Congress, Milan 7th-10th September 2022.
- Kunstfeld R. Poster 1692 presented at the 31st EADV Congress, Milan 7th-10th September 2022.
Klisyri® (tirbanibulin) Prescribing Information
Please consult the Summary of Product Characteristics (SmPC) before prescribing. Klisyri 10 mg/g ointment Active Ingredient: Each gram of ointment contains 10 mg of tirbanibulin. Each sachet contains 2.5 mg of tirbanibulin in 250 mg ointment. Excipients with known effects: Propylene glycol 890 mg/g ointment Indication: Klisyri is indicated for the field treatment of non-hyperkeratotic, non hypertrophic actinic keratosis (Olsen grade 1) of the face or scalp in adults. Dosage and Administration: Tirbanibulin ointment should be applied to the affected field on the face or scalp once daily for one treatment cycle of 5 consecutive days. A thin layer of ointment should be applied to cover the treatment field of up to 25cm2. Consult SmPC and package leaflet for full method of administration. Contraindications, Precautions and Warnings: Contraindications: Hypersensitivity to the active substance or to any of the excipients listed in section 6.1 of SmPC. Precautions: Contact with the eyes should be avoided. Tirbanibulin ointment may cause eye irritation. In the event of accidental contact with the eyes, the eyes should be rinsed immediately with large amounts of water, and the patient should seek medical care as soon as possible. Tirbanibulin ointment must not be ingested. If accidental ingestion occurs, the patient should drink plenty of water and seek medical care. Tirbanibulin ointment should not be used on the inside of the nostrils, on the inside of the ears, or on the lips. Application of tirbanibulin ointment is not recommended until the skin is healed from treatment with any previous medicinal product, procedure or surgical treatment and should not be applied to open wounds or broken skin where the skin barrier is compromised. Local skin reactions in the treated area, may occur after topical application. Treatment effect may not be adequately assessed until resolution of local skin reactions. Due to the nature of the disease, excessive exposure to sunlight (including sunlamps and tanning beds) should be avoided or minimised. Tirbanibulin ointment should be used with caution in immunocompromised patients. Changes in the appearance of actinic keratosis could suggest progression to invasive squamous cell carcinoma. Propylene glycol may cause skin irritation. Consult SmPC and package leaflet for more information. Fertility, pregnancy and lactation: No human data on the effect of tirbanibulin ointment on fertility are available. Tirbanibulin ointment is not recommended during pregnancy and in women of childbearing potential not using contraception. It is unknown whether tirbanibulin/metabolites are excreted in human milk. A risk to the newborns/infants cannot be excluded. Consult SmPC and package leaflet for more information. Adverse Reactions: Very common (≥1/10): Application site – erythema; exfoliation; scab; swelling; erosion (includes ulcer). Common (≥1/100 to <1/10): Application site – pain, pruritus and vesicles. Consult SmPC and package leaflet for further information. Legal Category: Ireland: POM Subject to prescription which may not be renewed (A). United Kingdom: POM Price: Ireland: Price to wholesaler United Kingdom: NHS Cost: £59.00 (excluding VAT). Marketing Authorisation Numbers: Ireland: EU/1/21/1558/001 United Kingdom: PLGB 16973/0043 Marketing Authorisation Holder:
Almirall, S.A., Ronda General Mitre, 151 08022 Barcelona, Spain Further information available from: Almirall Limited, Harman House, 1 George Street, Uxbridge, Middlesex, UB8 1QQ, UK. Date of First Issue: March 2025 Item code: UK&IE-TIRBA-2500001
UK and UK(NI)-Adverse events should be reported. Reporting forms and information can be found at MHRA https://yellowcard.mhra.gov.uk Adverse events should be also reported to Almirall Ltd. Tel. 0800 0087 399
IE-Adverse events should be reported. Reporting forms and information can be found at HPRA Website: www.hpra.ie Adverse events should also be reported to Almirall Ltd. Tel. +353 (0) 1431 9836
UK-KLI-2400001 January 2026
